We use dishwashers to make dishwashing more efficient, ensuring a spotless clean without the physical burden and saving valuable time. However, it’s important to remember that using the dishwasher alone isn’t enough — proper maintenance is key. One crucial aspect of dishwasher maintenance is the use of dishwasher salt, which plays a vital role in preventing limescale buildup and eliminating water streaks on your dishes. By softening the water, dishwasher salt allows the appliance to run more efficiently, protecting it from damage and ensuring your dishes come out spotless.
Cloudy Dishes from Hard Water?
Time to Check and Refill the Salt!
The water entering your dishwasher may be hard water, meaning it contains high levels of dissolved minerals like calcium and magnesium. Hard water can vary depending on your location, and areas with higher mineral content face more challenges. Over time, hard water can cause limescale buildup inside the dishwasher, leading to long-term damage and reduced performance. Softening the water before the main dishwashing cycle is crucial, and this is where dishwasher salt plays an important role.
In this article, we’ll explain how water softening works and the role of dishwasher salt.
The Problem and Solution: Hard Water and Dishwasher Salt
Hard water is water that contains high levels of dissolved minerals, primarily calcium and magnesium ions. These minerals lead to the formation of limescale buildup, which can clog the dishwasher’s internal components, reducing its efficiency and cleaning performance over time.
On the other hand, dishwasher salt is a specialized type of salt, mainly composed of sodium chloride. Its larger granules dissolve slowly, without blocking the water softening system in the dishwasher. By softening the water, dishwasher salt helps to prevent limescale buildup, ensuring your machine works efficiently and your dishes come out spotless.
HARD WATER

VS

DISHWASHER SALT
Comparison: Hard Water vs. Dishwasher Salt
| ASPECT | HARD WATER | DISHWASHER SALT |
|---|---|---|
| Mineral Content | Contains high levels of calcium and magnesium | Contains sodium chloride |
| Effect on Dishwasher | Leads to limescale buildup, affecting performance | Prevents limescale buildup by softening the water |
| Impact on Dishes | Leaves water streaks and cloudy dishes | Ensures spotless, clean dishes without streaks |
| Impact on Dishwasher | Reduces the lifespan and efficiency of the machine | Protects the dishwasher components and ensures optimal performance |
How Does the Water Softening Process Work?
When you use a dishwasher, the quality of the water it uses plays a crucial role in ensuring that your dishes come out clean and spotless. Hard water, which is rich in calcium (Ca²⁺) and magnesium (Mg²⁺) ions, can cause a number of issues. Over time, these minerals build up as limescale inside the dishwasher, leading to reduced performance, streaks on dishes, and potential long-term damage to your appliance.
Fortunately, the process of water softening can address these problems. Water softening is the process of removing calcium (Ca²⁺) and magnesium (Mg²⁺) ions from hard water and replacing them with sodium ions (Na⁺). This occurs inside an ion exchange resin in dishwashers, transforming hard water into soft water. The softened water prevents limescale buildup, protects the dishwasher’s internal components, and ensures that your dishes come out spotless.
What is Ion Exchange Resin and How Does It Work?
Ion exchange resin is a specialized material inside your dishwasher that plays a crucial role in softening water. The resin is packed with sodium ions (Na⁺), which are essential for the softening process.
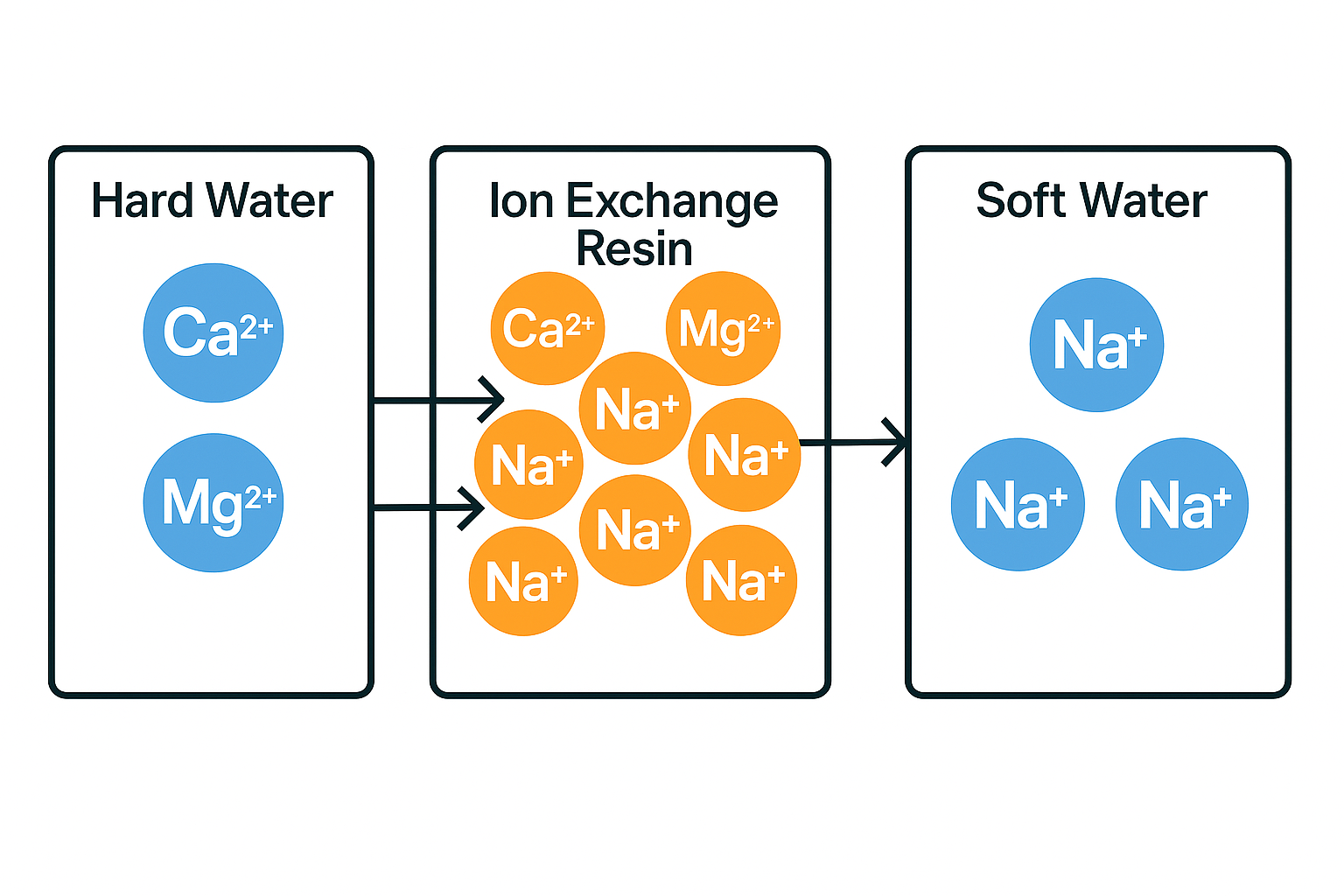
The Ion Exchange Process
When hard water enters the dishwasher, it first passes through the ion exchange resin. This is where the ion exchange process occurs. The calcium (Ca²⁺) and magnesium (Mg²⁺) ions from the hard water are exchanged for sodium ions (Na⁺) from the resin. As a result, the water exiting the resin is now softened and contains only sodium ions (Na⁺).
HARD WATER
→
→
ION EXCHANGE RESIN
HARD WATER
This soft water is then used for the main wash cycle in the dishwasher. The calcium and magnesium ions are flushed away or drained out of the system, leaving only soft water behind. Soft water helps prevent limescale buildup, ensuring the dishwasher remains in optimal condition and that your dishes come out sparkling clean.
The Role of Dishwasher Salt in the Water Softening Process
The ion exchange resin inside your dishwasher is charged with sodium ions (Na⁺), which are essential for the water softening process. But where do these sodium ions come from? The supply of sodium ions is replenished by dishwasher salt, and the most cost-effective source of sodium ions is salt (sodium chloride, NaCl).
Why is Regularly Refilling Dishwasher Salt Necessary?
As the ion exchange process takes place, the sodium ions are gradually used up. Since the sodium ions in the resin have a finite supply, they need to be recharged periodically. Without a regular supply of sodium, the resin can’t continue to soften the water properly. This is why regularly refilling dishwasher salt is crucial.
Dishwasher salt is specially formulated to replenish the sodium ions in the resin. By regularly refilling the salt reservoir, you ensure that the resin remains fully charged and continues to soften the water efficiently. This maintains the softening process and prevents limescale buildup, while also ensuring the longevity of your dishwasher.

In Summary

The sodium ions from the dishwasher salt are key to transforming hard water into soft water. This process not only protects your dishwasher from limescale buildup but also guarantees that your dishes come out sparkling clean. Regularly refilling the dishwasher salt ensures the water softening system remains effective, allowing your dishwasher to perform at its best and extending its lifespan.
To dive deeper into the essential components of dishwasher maintenance, be sure to check out our comprehensive guide on Dishwasher Detergent, Salt, and Rinse Aid.


